|
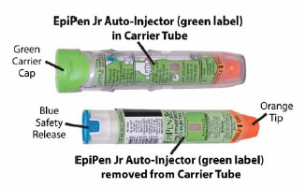
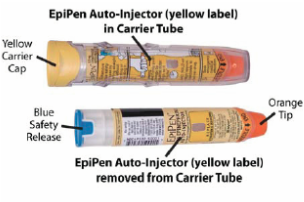
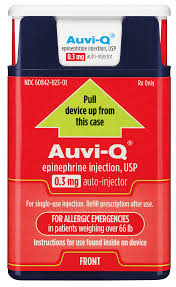
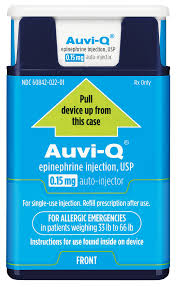
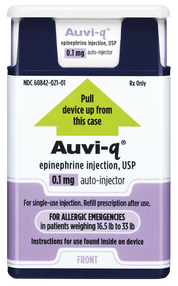
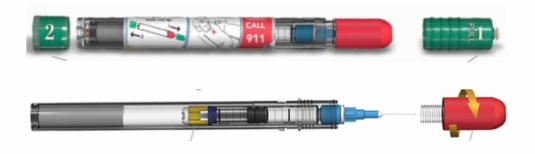
Initially published 2013, updated January 2020 Carrying epinephrine on day and overnight trips has become common practice for outdoor programs with staff trained in Wilderness First Aid (WFA) or Wilderness First Responder (WFR), or who have participated in an epinephrine training. Due to the seriousness of anaphylaxis, schools and other front country organizations are also likely to have staff trained in epinephrine administration. Additionally, it is common for individuals with known allergies to carry a personal prescription of epinephrine. Over the past several years a variety of manufacturers have produced epinephrine auto-injectors. It is important for individuals trained and responsible for the use epinephrine to be familiar with the variety of epinephrine auto-injectors. In addition, prescriptions other than epinephrine are also beginning to be placed in similar auto-injecting devices. Prior to use of any medication; confirm the type of medication, who it is prescribed to, and that it is within the expiration date. Dosage Most epinephrine auto-injectors are available in two dosages; 0.15 mg dose for individuals 33-66 pounds and 0.30mg for individuals above 66 pounds. The Auvi-Q has a third dosage size option that supports administration to infants and toddlers weighing 16.5-33 lbs. Device Usage See manufacturer websites, your doctor, or your program’s medical director for specific and detailed instructions for each of the auto-injectors listed below. EpiPen and EpiPen Jr. The EpiPen adult dose is in a device with a yellow label and the EpiPen Jr (child) has a green label. EpiPen works by removing the carrying case, removing the blue “safety” cap, pressing the application end of the device into the lateral (outer) aspect of the mid-thigh until the device clicks, hold in place for ten seconds, and dispose of device in an appropriate location. Both the EpiPen and EpiPen Jr. are designed to administer only one dose of epinephrine. Watch the EpiPen training video (leaves the LWM website and opens a new webpage) Adrenaclick Adrenaclick is administered similarly to the EpiPen. The additional step of removing the cap on the injection end of the device is needed to prepare for administration. The child dose has a purple colored carrying case and the adult dose has a black carrying case. Both child and adult devices administer one dose of epinephrine. Watch the Adrenaclick training video (leaves the LWM website and opens a new webpage) Auvi-Q Auvi-Q was recalled in 2015, but has since returned to the US market. Auvi-Q (Allerject in Canada) is a smaller pocket sized device. The device provides audio prompts to assist in administration of medication. The prompts work in the same way an AED provides direction for usage. The red packaged Auvi-Q is the adult dose, blue packaging indicates the dose for children weighing 33-66 lbs. and the purple package indicates a dose for infants and toddlers weighing 16.5-33 lbs.. The Auvi-Q is designed to administer a single dose of epinephrine. Watch the Auvi-Q training video (leaves the LWM website and opens a new webpage) TwinJect (Discontinued March 30, 2012) The TwinJect injector holds two usable doses of epinephrine. The first dose is administered similarly to the Adrenaclick. The second dose is accessed by removing the red cap on the injection side of the auto-injector. After removal of the cap, a syringe (same needle as first dose) is accessible for the administration of the second dose. Due to the multiple components to the TwinJect, it is highly advisable for individuals who have exposure to the device receive training with TwinJect training tools. Non-Epinephrine Auto Injectors Due to the ease of use, other medication (i.e., Alsuma, acute migraine medication) has been placed in auto-injectors. The device looks similar to epinephrine injectors and care needs to be taken to ensure medications are not confused at the time of administration. It has been suggested by members of the medical community that programs label non-epinephrine injectors as “NOT EPI” to provide fast identification during emergencies. This practice may reduce the potential of confusion or misadministration of medication by program staff. Storage Outdoor travel and recreation does not often support epinephrine's 68-77 degree F storage temperature range. Students participating in the LWM medical elective tested a variety of options for protecting temperature sensitive medications in environmentally dynamic environments. References
Gaudio F, Lemery J, Johnson D. Wilderness Medical Society roundtable report: recommendations on the use of epinephrine in outdoor education and wilderness settings. Wilderness & Environmental Medicine. 2010; 21:185-187. EpiPen (2020). Patient Information. Retrieved from EpiPen. website http://epipen.com Adrenaclick (2020). Patient Information. Retrieved from Adrenaclick. website https://www.adrenaclick.com/usage.php TwinJect (2013). Learn More About TwinJect. Retrieved from TwinJect. website http://twinject.com/auto-injector/learn-more.html TwinJect (2013). TwinJect Terms of Use. Retrieved from TwinJect. http://twinject.com/allergy-treatment-resources/notice-about-continued-availability.html Auvi-Q(2020). Patient Information. Retrieved from Auvi-Q. website http://www.auvi-q.com/media/pdf/Patient-Information.pdf Hawkins S, Weil C, Fitzpatrick D. Epinephrine Autoinjector Warning. Wilderness & Environmental Medicine.2012; 23:371-372.
0 Comments
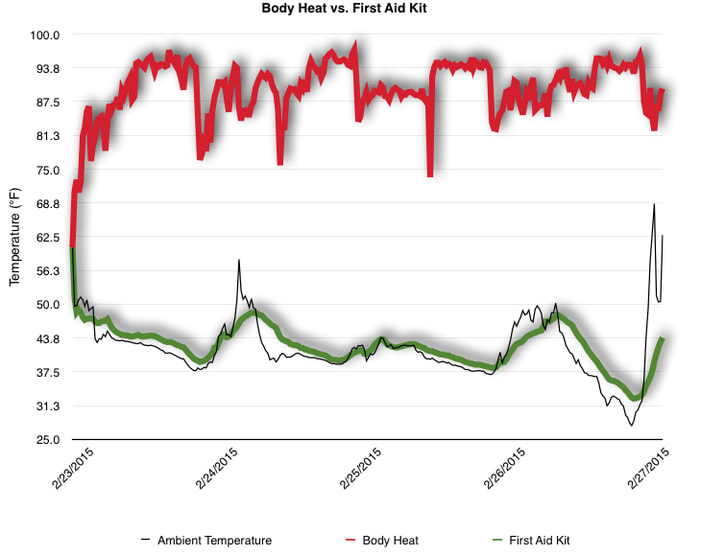
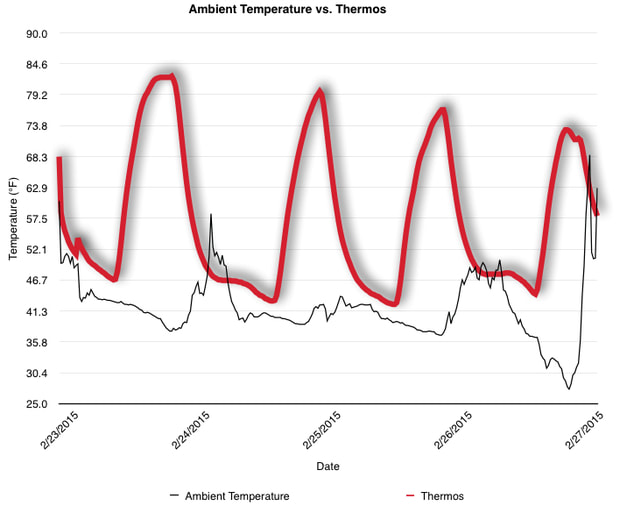
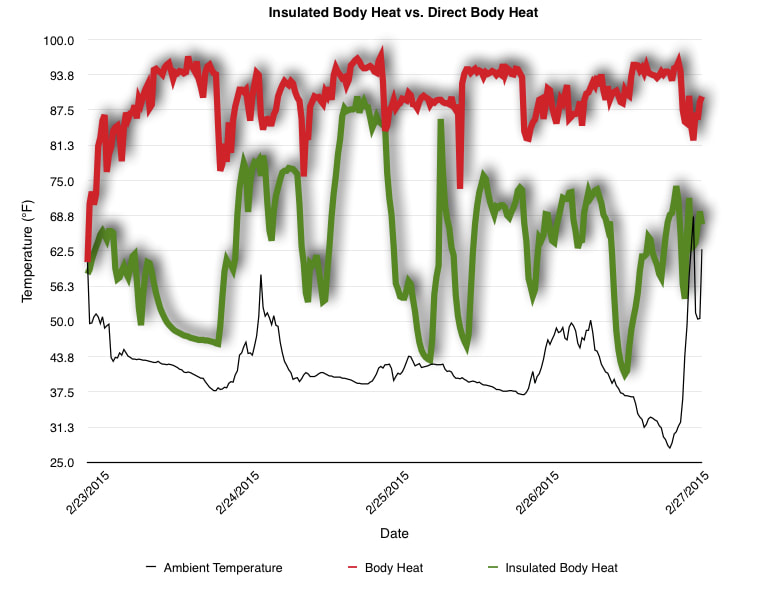
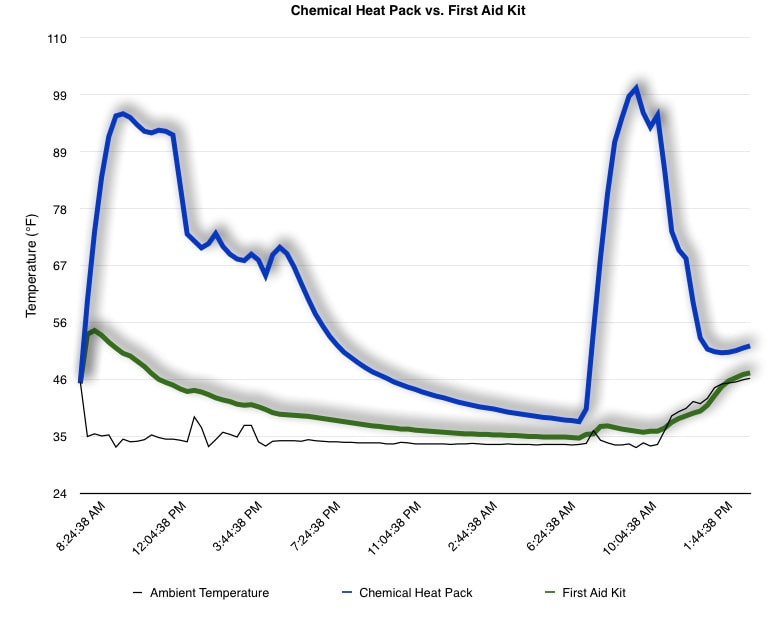
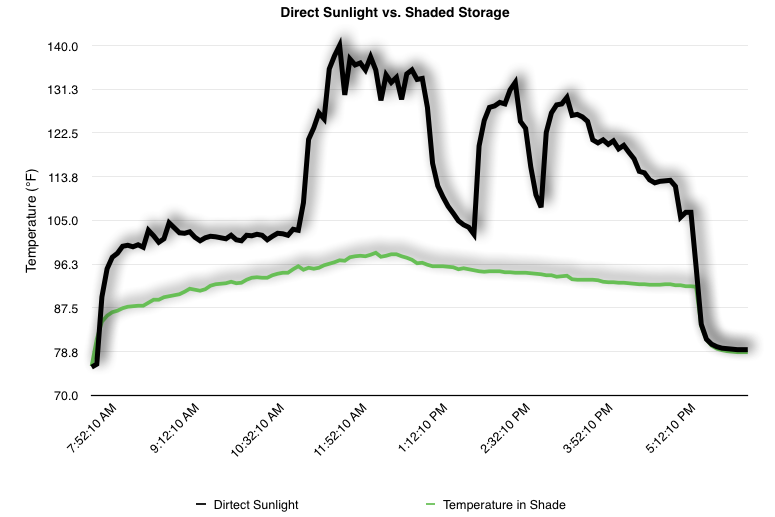
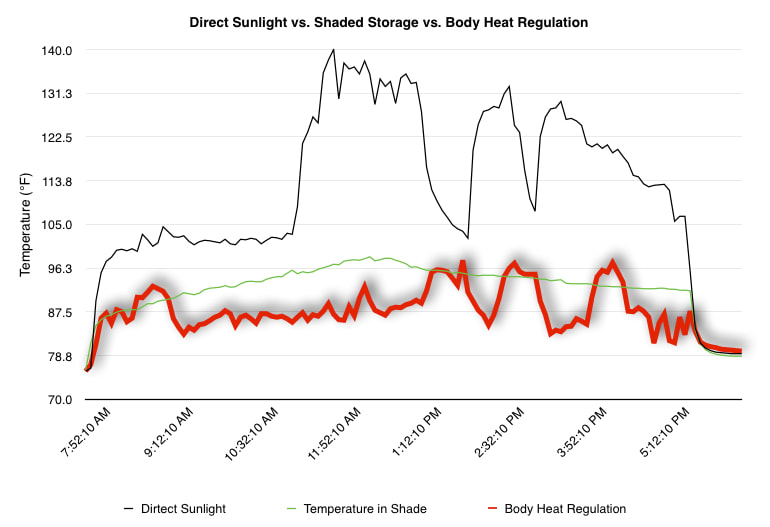
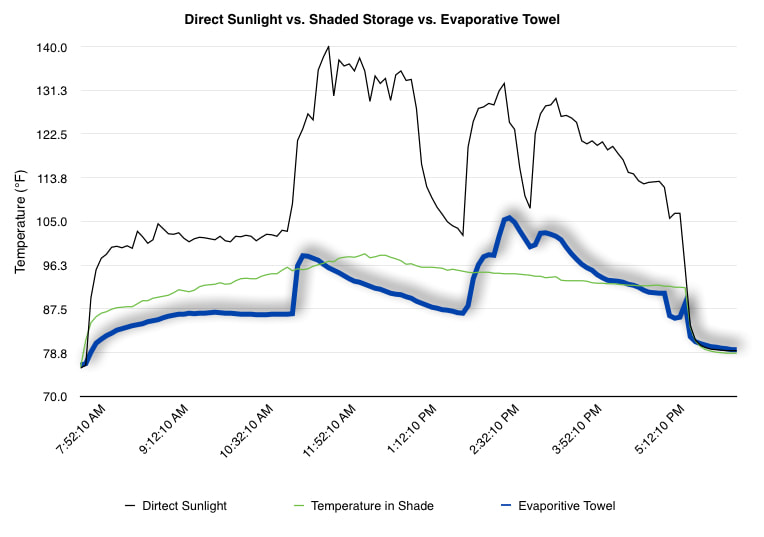
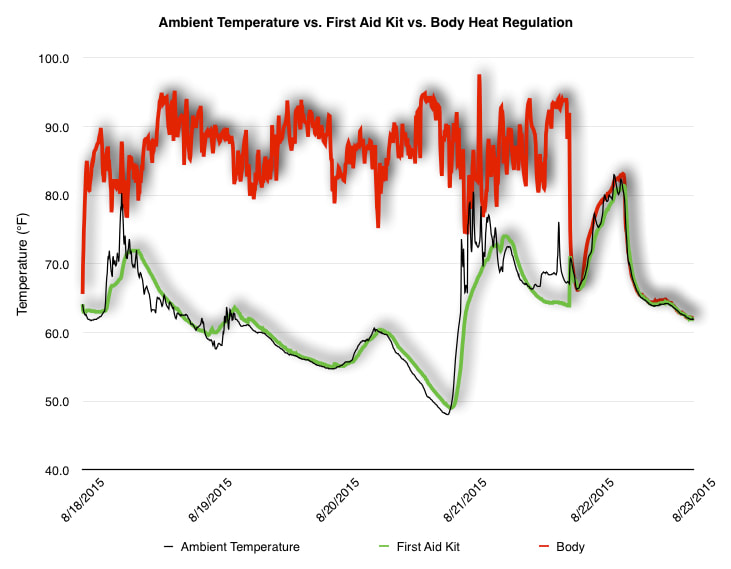
 EpiPen auto-injector EpiPen auto-injector In May 2016, the FDA released updated instructions for the usage of epinephrine auto injectors. The updates include shortening the length of time it takes to use the pen and highlight some of the potential risks associated with the use of auto injectors. Here is a condensed version of the recent changes as noted in the patient instructions for EpiPen and EpiPen Jr.: 1. Limit movement of the leg during injection This is especially important in children as they may jump or kick during the injection. Movement during the injection can cause bent or broken needles which may cause significant tissue damage and increase risk of infection. 2. Hold injection for 3 seconds Previous instructions for auto injectors have stated that the EpiPen must be held in position for 10 seconds in order to ensure the delivery of medication. Delivery of epinephrine does occur quickly due to the the force provided by the injection. Three seconds has been determined to be an adequate amount of time to hold the EpiPen in position, which may also significantly reduce the instance of injury as a result of bent or broken needles. 3. Potential of infection at injection site The updates also include information for patients to return to the hospital if signs of an infection present after injection. Although serious infection is not common, it is important to consider and to pay attention to the healing process at the injection site. The current recommendations add emphasis to only using the EpiPen on the lateral (outside) aspect of the thigh and not in the buttocks, intravenously, or in the hands and feet. Additional Epinephrine Auto Injector Information Revised May 2016 EpiPen and EpiPen Jr product information - Mylan Storage of Epinephrine in Remote Environments - LWM Identification of Epinephrine Auto Injectors - LWM Epinephrine: Medical & Ethical Necessity or Legal Nightmare - OutdoorEd.com Throughout 2015, LWM has been studying potential storage options for temperature sensitive medications due to the variable temperatures experienced in remote environments. The research initially began as a result of a letter written by Mylan Specialty L.P., the manufacturer of EpiPen, to the Editor of the Journal of Wilderness and Environmental Medicine. In the letter, the author shared Mylan’s concern about the range of temperatures experienced by EpiPens while in remote environments. The letter reiterated the storage guidelines from the product's label as 68℉ to 77℉ with excursions permitted to 59℉ to 86℉*. Reality is that there are few places that many of us like to recreate where temperatures can meet the storage needs of epinephrine and other temperature sensitive medications. In recent years, research has been done both regarding the effectiveness of epinephrine after multiple freeze-thaw cycles** as well as the post expiration effectiveness of the medication***. Although these papers generally support the thought that epinephrine is better used (as prescribed) than not used even when out of date and post thaw, in an ideal situation individuals carrying temperature sensitive medication will limit the temperature extremes these medications experience while in the field. Goal of the Study LWM wanted to investigate low tech and easy to operate solutions to maintaining a relatively temperature stable environment where temperature sensitive medication or expedition equipment could be stored while in austere environments. The market has limited tools designed to manage temperature in remote environments that are functional to carry while on an outing or expedition. Of the storage devices available, the majority are marketed as insulin storage devices for people managing diabetes. Most of these systems rely on chemical cooling packs that are refrigerated and provide several hours of temperature stability. One exception is the Frio Insulin Cooling Case (http://www.frioinsulincoolingcase.com/) which uses evaporative cooling to keep medication cool in warm environments. While these devices do work to keep medications cool, they are not able to increase the temperature of a stored med. Hypothesis Prior to the testing, assumptions were that the human body provided the most consistent temperature in the non-climate controlled environment of an expedition. The hypothesis of the tests was storage of the medication near the body would allow for a consistent temperature. The study focused on temperature nearest the torso under insulating layers but above a base layer (for wearer comfort). It was unknown if the body’s evaporative process would reduce heat experienced by the object in cool environments Testing LogTag data loggers were used to conduct the temperature assessment, with the logger being treated as the temperature sensitive item (TRIX-8, http://www.logtagrecorders.com/products/trix-8.html). The loggers were calibrated at the time of purchase. During testing periods temperatures were taken at intervals between 5 and 20 minutes for the duration of each test. Tests ranged from 10 hours to 5 days in length, dependent on the environment and activity completed during the study. During each study period, several temperatures were logged to gain an accurate comparison. Temperatures measured included ambient, temperature within a first aid kit on site, and temperature at base layer of clothing. In some environments additional management tools were assessed, including an insulated thermos and chemical heat packs for cold environments and evaporative towels for cooling in hot environments. Cold Weather Results Five Day Winter Canoe Expedition Students from the 2015 LWM Medical Elective tested their low tech solutions for managing temperatures of medication during the expedition component of the elective. Tests were conducted on the Mobile-Tensaw River Delta in southern Alabama over a five day period in February. Temperatures were recorded at 20 minute intervals. Temperature of the first aid kit remained near the ambient temperature shortly after the start of the expedition, but avoided the spikes due to the insulative properties of the bag the kit was stored in. Body heat offered temperature stability within a small margin. Daily drops in the temperature noted in the data derived from the device kept on the body are assumed to be during clothing change. One of the other solutions offered by the students in the elective was a high end thermos that was used to insulate the logger from the environment. The thermos was brought inside the student's sleeping bag each night resulting in a rise in temperature; however the warmth was not retained during the day. An additional system assessed was storing the logger in a clothing pocket. The logger was wrapped in a small fleece towel, placed in a plastic bag, and stored in a jacket pocket. The result was cooler temperature than experienced in the direct body heat logger, but varied much more in temperature. This is potentially due to the changes in type/weight of the layer being worn by the tester due to varied ambient temperatures and resulting in varied insulation of the logger from environmental conditions. Winter SAR Training A winter survival training for a North Idaho based search and rescue group provided the environment for the second cold weather test. Temperatures were recorded at 20 minute intervals during the two day assessment. The tester wearing the logger that assessed body heat was consistently active during the testing period, participating in activities ranging from hiking with a full pack to building emergency shelters. In addition to ambient, first aid kit, and body heat temperature assessments, a logger was placed with a chemical heat pack within a simulated first aid kit. The chemical heat pack was opened, activated, and then placed in a winter hat inside the first aid kit. Warm Weather Results Grand Bay, Mississippi During an August LWM Wilderness First Aid course in Grand Bay, Mississippi, outside temperature was assessed in the sun and shade at 5 minute intervals in order to provide a comparison to cooling techniques. The logger in the direct sun was moved when it became shaded by shadows as the day passed. The dramatic decreases in temperature are a result of this short term shading. One of the test loggers was worn by a LWM instructor as a necklace in similar fashion to the tests completed in cold weather. The results of the test showed a noticeable cooling as compared to either the shade or direct sun exposure. Due to the nature of the course there were times where the instructor went inside while wearing the logger. Two of these times occurred in the afternoon and are noticeable drops. In addition to body heat regulation, the temperature inside an evaporative cooling towel (http://www.froggtoggs.com/cooling/the-chilly-pad/chilly-pad.html) was taken while placed in the sun. The cooling towel provides a similar environment to the Frio insulin cooling device mentioned above. Warming spikes in temperature are noted in the chart when the towel became dry. Cooling after the spikes was facilitated by dipping the towel in lukewarm water. Five Day Summer Backpacking Trip The final test in this series was completed on a five day backpacking trip on Michigan’s Isle Royale National Park. Assessments of ambient, body, and first aid kit were recorded at 15 minute intervals during four of the five days. During the test the tester was backpacking with a approximately 40 pound backpack at a moderate pace. The logger was kept in the tester's sleeping bag each night, but not attached to his body. Discussion There is no perfect solution to temperature management in remote environments, but with some effort, a level of temperature stability can be achieved. Using epinephrine as an example, there are several ways to work toward maintaining the 59-86 ℉ temperature range. Although slightly warmer than product labels recommend, regulation with body heat appears to be the best solution for cold weather trips. In mild temperatures as experienced during the Isle Royale trip, the best storage location, per the data received from the loggers, was the first aid kit. However, when planning for unknown temperatures, this study showed that body heat provided the most consistent temperature through a range of environmental temperatures. In hot weather environments, body heat storage temperatures remained relatively consistent, although higher than the EpiPen label recommends. The cooling towel proved the most comfortable option in the heat and was relatively consistent. However, as noted in the above study, there is the potential increased temperature if the evaporative material gets too dry. There are commercially available options designed specifically for the storage of epinephrine on the body that would provide relative comfort while on extended trips, including products such as the WaistPal (http://www.omaxcare.com/WaistPal.html). Temperature management is not the outright goal of these devices, but they would offer a comfortable, more temperature stable solution based on the findings listed above. A novel and low cost solution for an individual carrying multiple medications (e.g. a SAR medic or expedition doctor) could involve placing medication in a small organizer case and keeping the case in a holster device similar to those used for avalanche beacons. Avalanche beacon harnesses offer improved comfort while wearers are active and work well even when wearing a backpack. This would be more favorable then wearing a necklace, but does comes with space limitations. If traveling in hot environments, the medication organizer can be secured to the outside of a pack using a daisy chain or tactical MOLLE system after being wrapped in a evaporative towel. Conclusion This informal study begins a foundation for knowledge about the temperatures experienced by temperature sensitive medications in remote environments. The information above can be used to help make informed decisions on the storage of temperature sensitive medications in environments with variable temperatures. Additional considerations regarding temperature and storage include making storage decisions based on changing weather systems and temperature changes due to changes in elevation. Regardless of where medication is stored, it is most important to make sure that those who need to know where they can find it efficiently if it is needed. WM would like to thank to the following individuals for participation in the temperature study: Katie Cartier Carl Engelke Ben Dowdy Dave Ramsey Chris Buckley Wes Edmonds Katie Laycock David Nesbitt Tim Parker Lara Sancin Sources:
* Wolf, Ray A. (2014). Regarding the Use of Epinephrine Auto-injectors in Remote Settings. Wilderness & Environmental Medicine, 25 (4), 490. **Beasley, Heather, et.al., (2015). The Impact of Freeze-Thaw Cycles on Epinephrine. Wilderness & Environmental Medicine, 26(1). 94 ***Simons, F.E., et. al., (2000) Outdated EpiPen and EpiPen Jr Autoinjectors: Past Their Prime? The Journal of Allergy and Clinical Immunology 105(5). 1025-30. |
Archives
December 2020
Categories
All
|








 RSS Feed
RSS Feed
